Frequently Asked Questions
How does TROVO work?
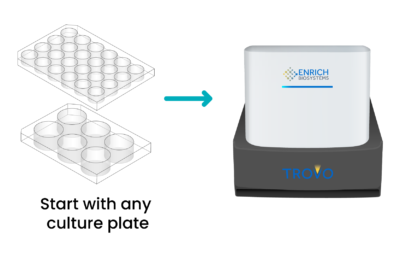
Virtually any culture condition including co-culture (for example, T cells and tumor cells) for up to 36,000 conditions. Each indexed well can be imaged at desired timepoints, including at initial seeding to provide visual confirmation of single-cell clones.
What is the microwell-based capture mode?
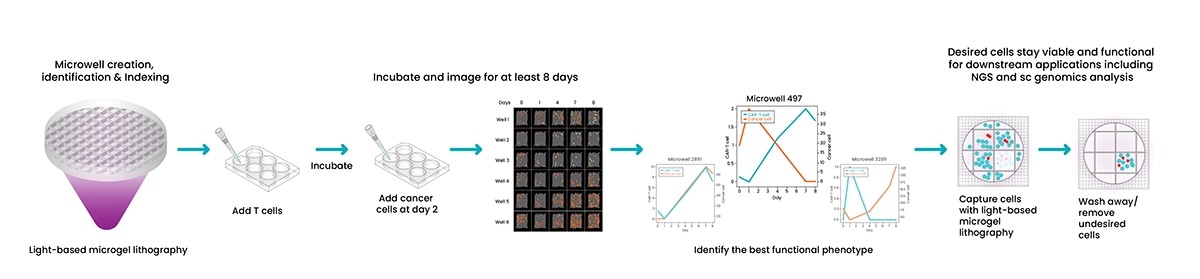
Microwell-based capture mode employs creation of micron-sized wells to enable high-throughput, long-term cell culture experiments.
-
Cell culture plates with Enrich hydrogel are loaded into TROVO.
1
-
Using a patented, light-based microgel lithography and LCD dynamic mask, TROVO can create up to 24,000 custom microwells of any size and shape in a 6-well culture plate.
2
-
Wells are visually identified and indexed for downstream data collection and analysis.
3
-
Cells are then seeded into the wells, imaged, and allowed to culture for up to weeks. During incubation, additional cells can be added to test functional interactions and phenotypes.
4
-
Each indexed well can be imaged at desired timepoints, including at initial seeding to provide visual confirmation of single-cell clones. Indexed images can be analyzed to provide cell kinetics and more accurate profiling data to identify the best functional phenotype.
5
-
Following visual identification, TROVO can capture and maintain viability and functionality of the desired cells for downstream applications including NGS and single-cell genomics/transcriptomics analysis. Undesired cells are simply aspirated away from the captured cells.
6
What is the free space capture mode?
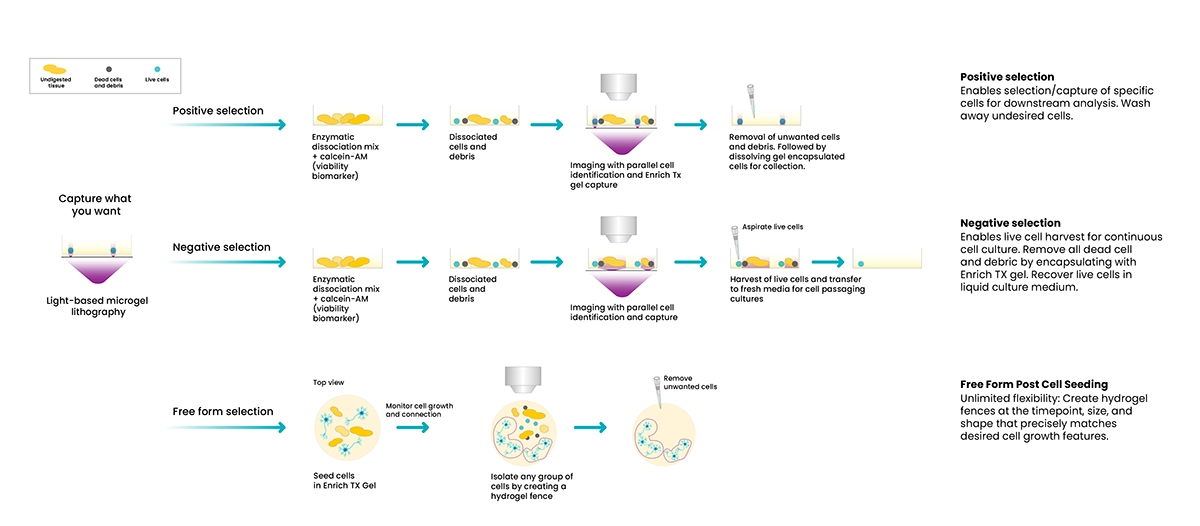
Free space capture mode enables you to capture and select the cells you want using an open selection tool with Enrich hydrogel. With this mode, you have the flexibility to perform positive, negative, and free form selection.
- Positive selection enables capture of desired cells while undesired cells, dead cells, and debris are washed away.
- Negative selection enables capture of undesired cells, dead cells, and debris. Live, desired cells are aspirated and transferred to a new culture dish or harvested for downstream applications.
- Free form selection is a limitless, precise selection tool that enables you to isolate cells or networks of cells within the culture plate.
What are the arrayed CAR T CRISPR screens?
Arrayed CAR T screens involve targeting one genetic modification per well in a multiwell format. Common arrayed screens include genetically modifying CAR T cells, purifying for each individual CAR T modification, and inputting each individual CAR T variant into individual wells of a 96 or 384 well plate.
The TROVO platform from Enrich Biosystems enables high-density, microfluidics-free CRISPR screening by creating thousands of hydrogel-based microwells to array an entire CRISPR library in one well of a microtiter plate, eliminating the need to purify for each CAR T variant.
TROVO then images and analyzes these microwells for activity and finally isolates and retrieves only cells with the desired phenotype. Those retrieved cells, commonly T cells with persistent cytotoxicity, can then be sequenced to identify genes correlating to T cell persistence.
Are there any alternatives to FACS? I have very few cells but need to isolate them into different populations.
FACS is a very powerful technique which very quickly isolates individual cells based on unique biomarkers conjugated to fluorescence molecules. A FACS run requires a minimum input of hundreds of thousands of cells, which limits FACS suitability for small sample sizes like small tumor biopsies, and has limited utility in detecting rare event biology, like isolating TILs from a blood sample.
TROVO, a microfluidics-free cell isolation and retrieval system from Enrich Biosystems, requires only thousands of cells and can isolate rare event cells, including tumor reactive T cells such as TILs, without any need for biomarkers. TROVO’s function-first screening method, based on microculture movie analysis, can identify and isolate tumor reactive T cells without any need to first screen peptides to discover unique neoantigens.
Are there any new techniques to isolate T cells from solid tumors?
TROVO, developed by Enrich Biosystems, can create more than 20,000 microcultures in a standard 6-well microtiter plate by precisely printing small hydrogel walls via microgel lithography. These hydrogel microwells can be used to co-culture individual T cell clones against solid tumors, including melanoma, breast, lung, brain, and many others. TROVO then quickly analyzes each microculture with AI-driven, deep-learning cell segmentation models to identify potent T cells that have therapeutic potential accurately.
Based on this analysis, TROVO, using the same microgel lithography technique that creates the hydrogel microwells, precisely prints a second gel that encapsulates only potent T cells. Those encapsulated cells can either be sequenced or expanded.
Other single-cell isolation techniques, which are dominated by microfluidics and droplets (including FACS) and optofluidics (including optical trapping), have difficulty retrieving T cells from solid tumor co-cultures. TROVO enables gentle recovery of viable, active T cells directly from complex tumor co-cultures for sequencing or expansion.
Can TROVO be used for downstream single-cell RNA sequencing (scRNA-seq)?
Yes. TROVO is compatible with downstream single-cell RNA sequencing workflows, including both 10x Genomics and Smart-seq approaches. Cells are retrieved gently and without fluidic pressure, preserving viability and RNA integrity.
The platform supports rare cell enrichment from tissue or tumor digests, making it particularly useful for scRNA-seq experiments where sample size is limited and phenotype-driven selection is critical.
How does TROVO fit into my existing cell discovery or screening workflow?
TROVO is designed to plug into existing discovery pipelines by offering a front-end, phenotype-based screening layer. It allows researchers to screen thousands of cell variants or co-cultures in parallel without needing to pre-select markers or targets.
TROVO supports functional assays such as cytotoxicity or persistence, and retrieved cells can then be labeled, expanded, or sequenced using downstream methods already in place in most labs.
How can I screen a large number of cell variants without pre-selecting targets?
TROVO allows researchers to perform large-scale cell discovery and screening without needing to pre-select markers or antigens. Its microwell-based format supports thousands of parallel microcultures per plate, enabling unbiased screening for functional phenotypes like cytotoxicity or persistence. This eliminates the need to narrow your screen before starting, helping you capture rare or unexpected cell behaviors that might be missed in traditional marker-based workflows.
What types of cells or assays are compatible with TROVO?
TROVO supports a wide range of cell types and experimental designs, including suspension and adherent cells, tumor co-cultures, and immune-tumor interaction assays.
The platform has been used successfully for CAR-T, TCR-T, TIL, and B cell screening, as well as for tissue-digested samples in scRNA-seq prep workflows. TROVO is highly adaptable due to its customizable microwell environment and non-fluidic retrieval system.







